At 200 X 400 micrometres this zircon crystal extends the limits of our understanding of early Earth back to 4.4 billion years ago, a mere 100 million years after the Earth was formed and only 160 million years after the Solar System formed. This discovery pushes back the date for when Earth first had a solid crust and weakens the theory that at this time the Earth was hot and entirely molten. It seems the conditions were much milder much earlier than we thought. There are two methods of dating these minerals, via radioactive dating methods and using atom probe tomography. The research group who dated this sample used atom probe tomography, but I am going to talk about radioactive dating methods.
Zircon crystals are brilliant for radioactive dating because of two reasons. Firstly they're tough, they last a long time and can withstand an amount of tectonic pressures. Secondly, and most importantly, they form with radioactive uranium in their structures AND they do not form with lead in their structures. Why is this significant? Radioactive uranium degrades into lead. So we can say that any lead present in the crystal is due to the decay of the uranium and nothing else. Zircon crystals are special in another way. They form with two different radioactive isotopes of uranium which both degrade at their own independent rates into two differing isotopes of lead.
Radioactive decay happens with precise timing. We know how radioactive decay works and at what rate it happens at. It's an exponential decay that is as regular as clockwork. Whether this happens over billions of year, hours, minutes or microseconds, all radioactivity follows exponential decay that is given in a simple mathematical expression.
So essentially zircon crystals are like clocks that were set to zero when they solidify from molten rock. Actually more like two individual clocks in each crystal because of the two different radioactive decays. All we need to do is to look at the ratio of uranium to lead to tell what time it is, just like looking at the two hands of an analogue clock. And the beauty is that we have two clocks running simultaneously and confirming each other.
So you start off at time zero with 100% of the parent isotope. This degrades at an exponential rate to a daughter isotope. For example:
We can say how fast each radioactive decay occurs by a concept called 'half-life'. A half-life is defined as the time when half the amount of the parent isotope has decayed into the daughter isotope. So at time zero there is 100% parent isotope and at the half-life there is 50% parent isotope and 50% daughter isotope. And because this decay is exponential, at 2 times the length of the half-life the parent isotope is at 25% and the daughter at 75% and so on.
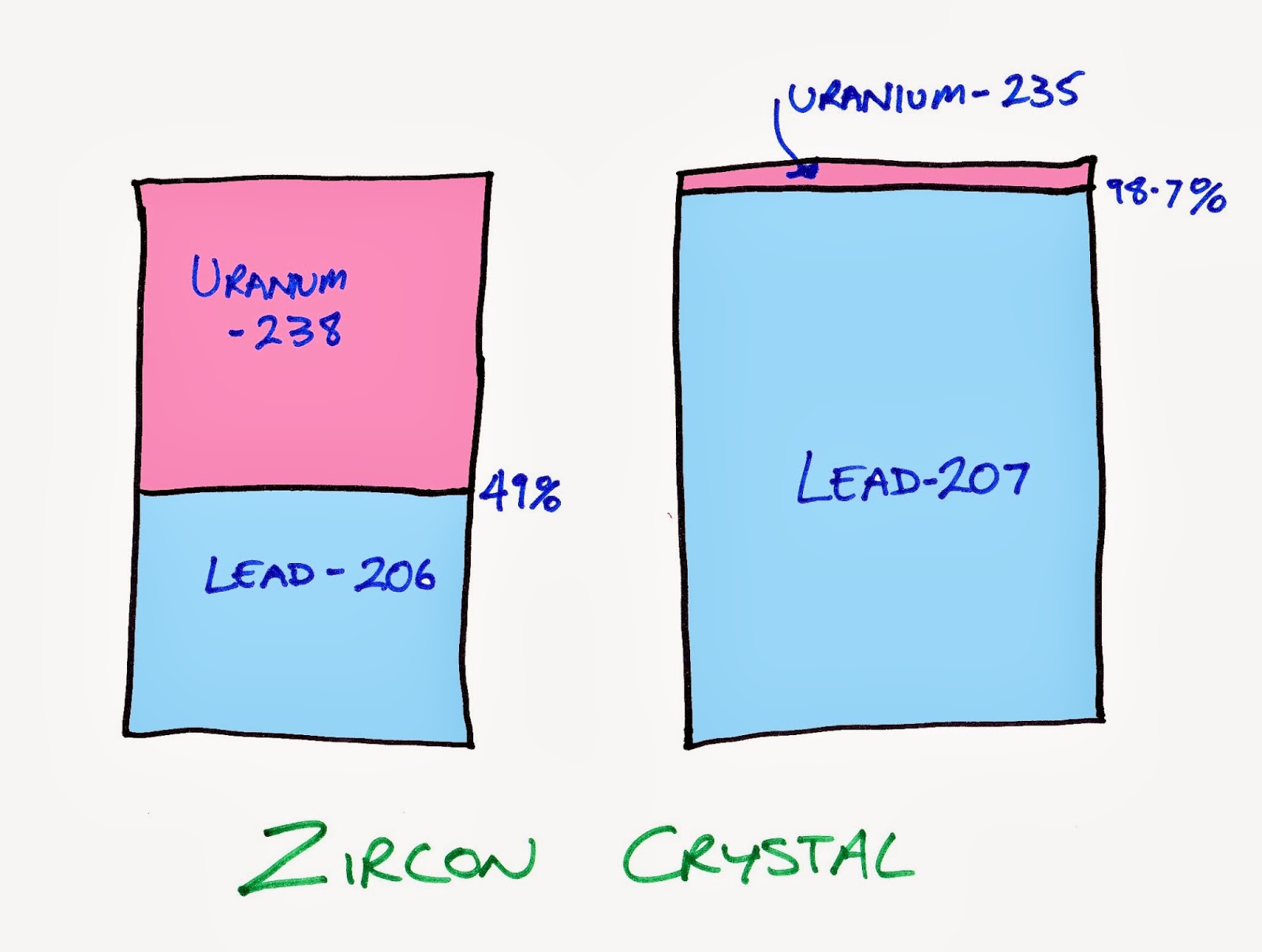
Each radioactive decay happens at its own rate. For example the half-life of uranium-235 is 704 million years, the half-life of uranium-238 is 4.5 billion years, while ruthenium-106 is 1 year, carbon-11 is 20 minutes and lithium-12 is 10 nanoseconds. So for the two uranium decays in zircon you can see that the decay from uranium-235 happens at a much faster rate than that of uranium-238.
Scientists trying to age a zircon crystal look at how much of uranium-238 versus lead-206 there is and how much uranium -235 versus lead-207.
You can use these ratios to back calculate ages of the crystal. Looking at uranium-238 there is nearly a 50-50 mix of parent to daughter isotopes, so therefore it is just under the age of its half-life of 4.5 billion years. With the uranium-235 you can see that most of it has decayed to lead-207 and there isn't much uranium left at all. It has gone through many half lives. In fact we can calculate it has gone through 6.25 half-lives and 6.25 multiplied by 704 million years is 4.4 billion years. So you can see how the correlation of the ages calculated of these isotopes are a powerful tool with both results pointing to 4.4 billion years or so.
The same method of looking at the ratio of parent to daughter isotopes is used for all radioactive dating. The other type of dating that you may have heard of is radiocarbon dating or carbon-14 dating. This method looks at the amount of carbon-14 in a sample which could be some bones of animals or humans or trees or other living things. Because all living things have a large amount of carbon in them, some of the carbon is naturally occurring carbon-14 (as opposed to the normal carbon-12). When a living thing dies it does not absorb any more carbon into its system and the carbon-14 that remains decays. So the amount of carbon-14 left in a biological sample tells us how long ago it died. The half-life of carbon-14 is 5,730 years and so if we find half the expected carbon-14 in a sample is must be around the age of 5,730 years. This method is limited to around 60,000 years or 10 or so half-lives. After this time there is only 0.1% of the parent isotope left and the error becomes too large to be reliably date a sample.
So you can see how radioactive decay is a useful tool to date objects. You cannot use every type of radioactive decay out there to age objects, it is limited to systems where you know the concentration of one isotope at time zero. But luckily there are several systems where this occurs and knowing this fact gives us a glimpse into the distant past.
Just for the hell of it here is a zircon crystal from my own collection. It's probably nowhere near as old as 4 billion years as it is large and was from a location in the Northern Territory that is not renowned for it's age.





No comments:
Post a Comment